继肺癌、胸腺癌后,HLX43广谱抗肿瘤潜力持续验证
入选ESMO Asia优选论文口头报告,HLX43晚期宫颈癌II期临床数据首发亮相
初步临床疗效优异且安全性可控,总人群ORR、DCR分别为41.4%及82.8%,其中3mg/kg剂量组ORR和DCR高达70.0%和100%
2025年12月5日,在2025年欧洲肿瘤内科学会亚洲年会(ESMO Asia)上, 复星医药 子公司 复宏汉霖 PD-L1 ADC HLX43用于复发/转移性宫颈癌的II期临床研究数据以优选论文口头报告形式首发亮相,展现了令人鼓舞的初步疗效,继非小细胞肺癌、胸腺癌之后,再度印证了该产品在实体瘤领域的广谱治疗潜力。


宫颈癌数据报捷,广谱潜力再验证

宫颈癌(CC)、卵巢癌等妇科恶性肿瘤给女性健康造成了严重威胁 [1-2],尤其晚期患者预后较差。一线联合疗法治疗后,约30%的晚期宫颈癌患者出现疾病复发[3],其5年生存率约为17%[4],包含多西他赛在内的二线化疗客观缓解率(ORR)仅约为13.2%[5],尽管PD-1抑制剂及靶向组织因子(TF)的抗体偶联药物(ADC)Tisotumab vedotin已获批上市,但其治疗既往治疗失败的复发转移性宫颈癌疗效也有限,ORR多不到18%[6-9],亟需更高效、临床获益更为显著的治疗新选择。
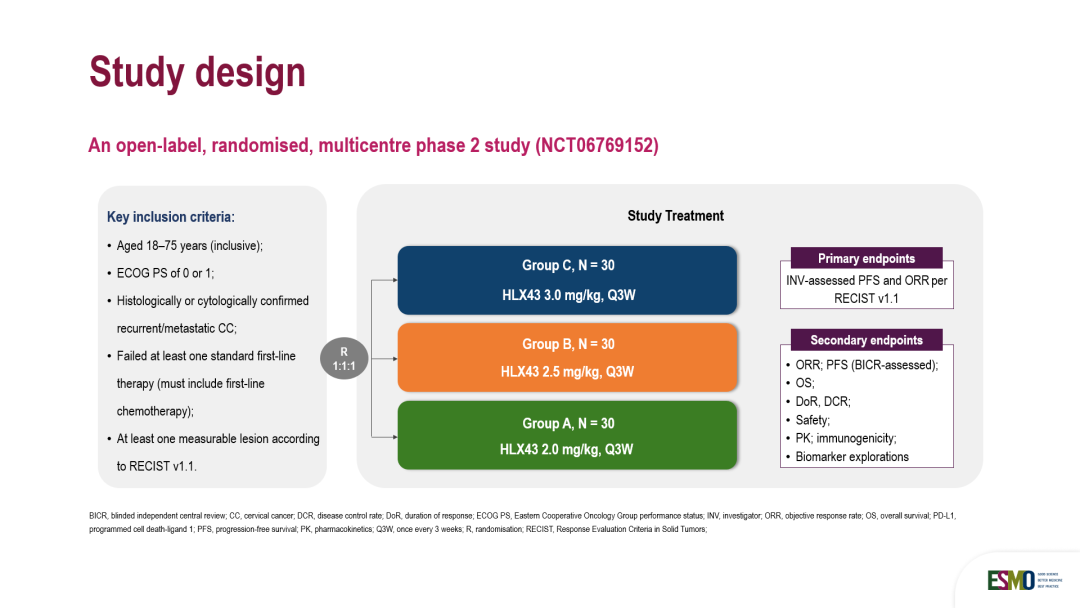
本次发布基于一项开放、随机、多中心的II期临床研究,由 山东省肿瘤医院 于金明院士牵头开展。经组织学确诊为复发/晚期宫颈癌(CC)且既往接受过标准一线治疗失败、不耐受或禁忌的患者,按1:1:1比例随机分组,每3周接受一次剂量为2 mg/kg、2.5 mg/kg或3 mg/kg的HLX43治疗。主要终点为研究者根据RECIST v1.1评估的客观缓解率(ORR)和无进展生存期(PFS)。次要终点包括其他有效性指标、安全性、药代动力学、免疫原性和生物标志物探索。
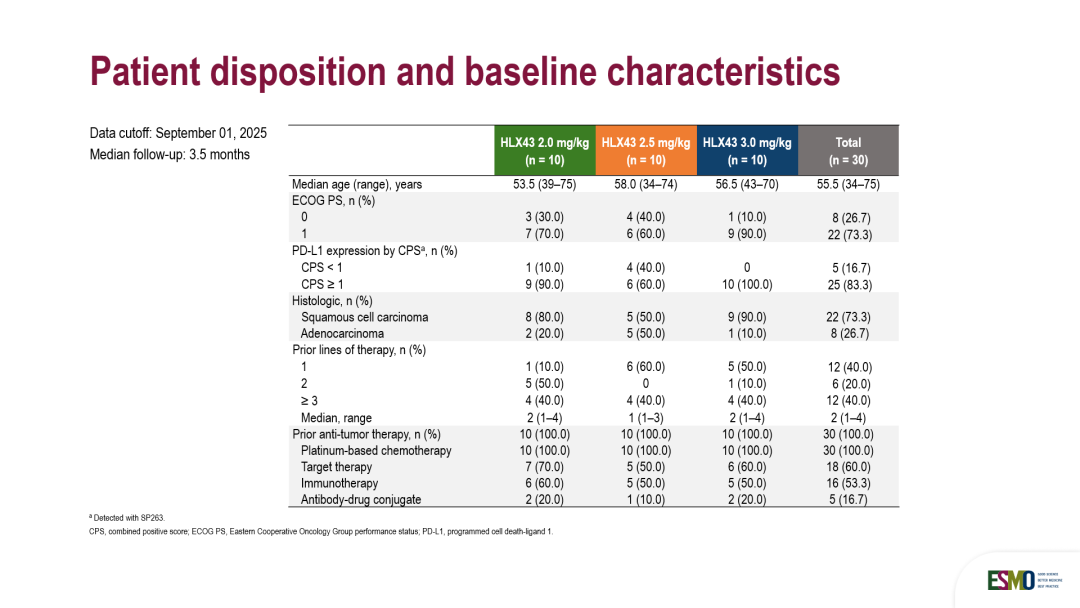
截至2025年9月1日,本研究共纳入30例患者,随机分配接受剂量为2 mg/kg(n=10)、2.5 mg/kg(n=10)和3 mg/kg(n=10)的HLX43治疗。其中超过80%的患者PD-L1综合阳性评分(CPS)≥1。患者既往接受肿瘤治疗的中位线数为2.0(范围1–4线)。全部患者接受过铂类药物化疗,60%的患者接受过靶向治疗,约50%患者接受过免疫治疗,中位随访时间为3.5个月。
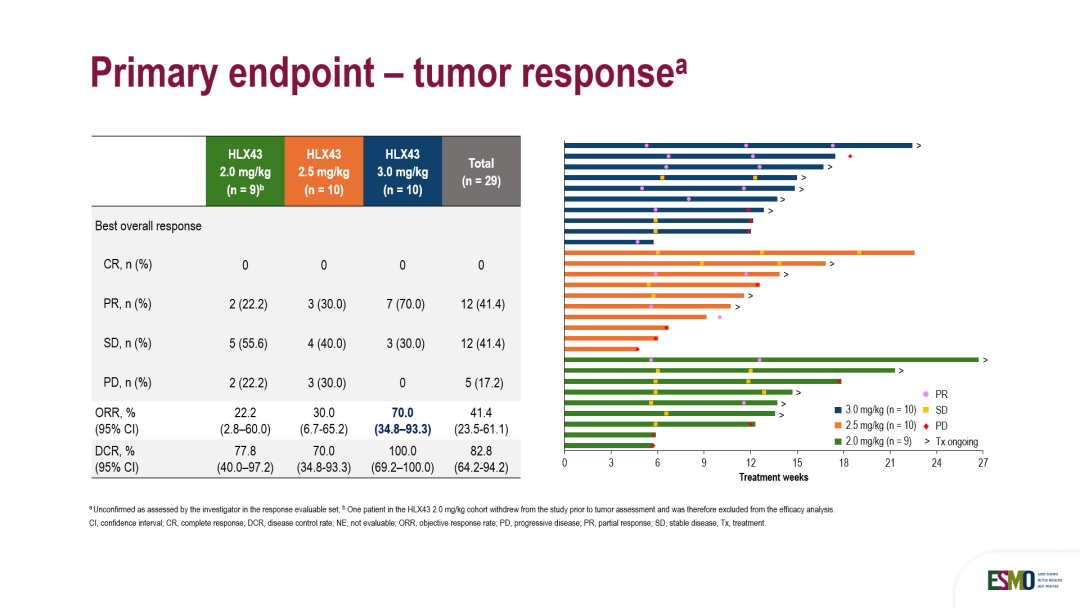
在29例可评估疗效的患者中,研究者评估的客观缓解率(ORR)为41.4%,疾病控制率(DCR)为82.8%。其中,3 mg/kg剂量组的ORR和DCR为70.0%和100%,患者中位PFS尚未达到。
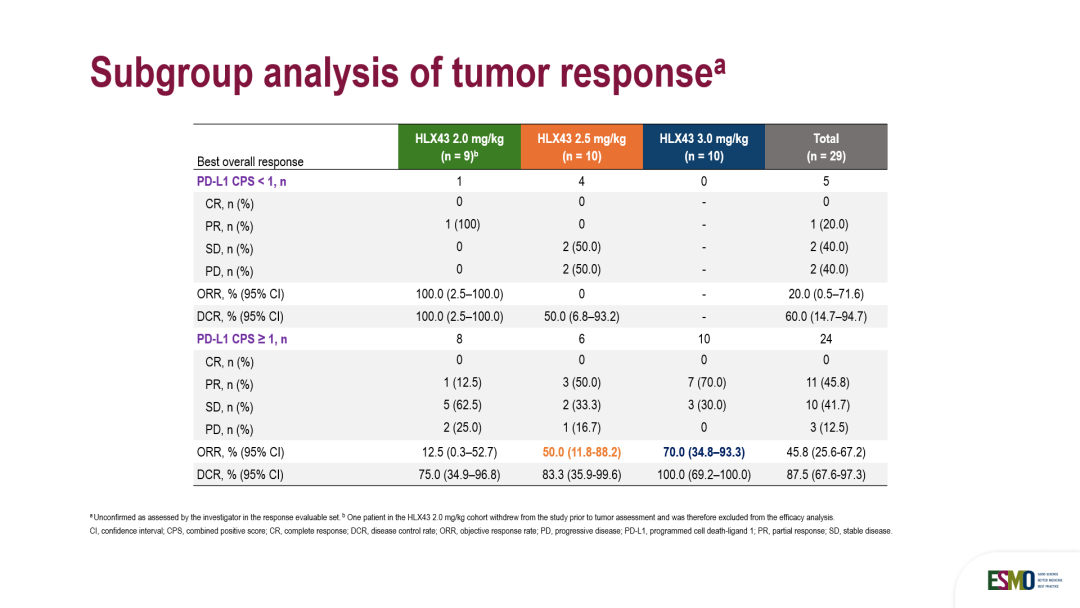
亚组分析显示,HLX43在CPS≥1的PD-L1阳性晚期宫颈癌患者中初步疗效优异,ORR和DCR分别达到 45.8%和 87.5%,并在CPS<1的PD-L1阴性患者中展现了一定疗效(1例2mg/kg剂量组患者达到PR),但鉴于此次纳入的患者基数较小,且CPS ≥ 1占比较高(超过80%),仍需待后续更大样本量的随机对照研究,以明确HLX43在PD-L1阴性亚组中的确切疗效。
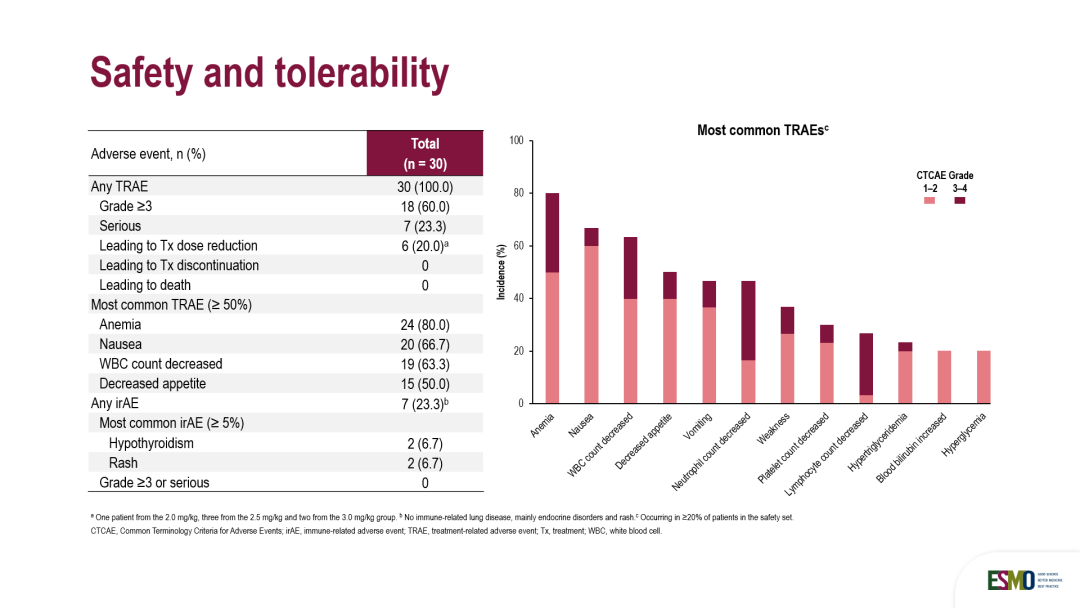
安全性方面,18列(60.0%)患者报告了≥3级治疗相关不良事件,主要为可管理的血液学毒性,常见包括为中性粒细胞计数降低(30.0%)、贫血(30.0%)和淋巴细胞计数降低( 23.3%),6例(20.0%)患者因TRAE导致剂量减少,无TRAE导致的患者停止治疗(导致停药的TRAE发生率为0%),且无TRAE导致的死亡。其中,7例(23.3%)患者发生免疫相关不良事件(irAE),主要包括甲状腺功能减退症等内分泌异常、皮疹等。无患者报告≥3级免疫相关不良事件(irAE),提示了HLX43的IO疗效,且免疫相关毒性温和,具有可控的安全性特征。
整体来看,HLX43 在复发/晚期宫颈癌(CC)的后线治疗中展现出可控的安全性和令人鼓舞的初步疗效,尤其在3.0 mg/kg 剂量下疗效更为显著,值得进一步研究。
肺癌疗效优异,重磅实力定基石
HLX43是一款潜在同类最优及疾病领域最优的广谱抗肿瘤ADC,兼具免疫检查点阻断与载荷细胞毒性的双重作用机制。临床前研究显示,HLX43在PD-1/PD-L1单抗耐药的非小细胞肺癌、宫颈癌、食管鳞癌等多个瘤种中展现出治疗潜力,且耐受性良好。其I期临床数据于2025美国临床肿瘤学会(ASCO)年会及2025 世界肺癌大会(WCLC)上先后发布,在NSCLC等实体瘤中展现出“高效、低毒”的显著疗效,尤其在NSCLC的治疗上,HLX43展现了全人群覆盖的潜力,对于鳞状/非鳞状NSCLC,有无EGFR突变、有无脑转移、PD-L1阳性/阴性的NSCLC患者人群都具有疗效,不依赖生物标志物筛选。
目前,公司正全力推进HLX43临床开发进程,在全球入组超过500例患者,其中NSCLC患者超过270例。随着这一适应症的后线疗效逐步得到验证,未来公司计划开展更多HLX43在肺癌领域的III期临床研究,全面覆盖HLX43头对头对比多西他赛的二线临床研究、HLX43用于NSCLC的一线治疗及新辅助治疗方案等。同时,HLX43作为全球首个布局胸腺癌的PD-L1 ADC,其国际多中心临床研究在中、美、澳等国家同步推进。2025年10月,基于HLX43在胸腺癌后线治疗中优异的初步疗效,该产品获得FDA孤儿药资格认定,有望填补这一罕见高侵袭癌种 ADC治疗的空白。
肺癌和胸腺癌之外, 复宏汉霖 已累计开展约10项HLX43治疗多项实体瘤中的临床研究,广泛覆盖宫颈癌等晚期妇科肿瘤、食管鳞癌、头颈鳞癌、鼻咽癌、结直肠癌、胃癌/胃食管交界部癌、胰腺导管腺癌、肝细胞癌等。其中,HLX43在食管癌、鼻咽癌、胃癌等实体瘤中的概念验证数据也将在ASCO GI、ASCO、ESMO等大会上陆续读出。单药之外,基于HLX43展现出的IO疗效,公司积极探索HLX43与其他多元分子如公司自研创新抗EGFR单抗HLX07的联合治疗潜力,不断挖掘和最大化该产品在临床中的应用价值。
未来, 复宏汉霖 将持续聚焦患者未满足的临床需求,立足于HLX43等核心创新管线,不断放大产品的差异化治疗潜力,加速推动更大临床价值的释放,为全球患者带来更具突破疗效的治疗方案。
【参考文献】
[1] Siegel RL,Giaquinto AN,Jemal A.Cancer statistics,2024.CA Cancer J Clin.2024;74(1):12-49.doi:10.3322/caac.21820
[2] Han B,Zheng R,Zeng H,et al.Cancer incidence and mortality in China,2022.J Natl Cancer Cent.2024;4(1):47-53. Published 2024 Feb 2.doi:10.1016/j.jncc.2024.01.006
[3] Gennigens C, et al. Expert Rev Anticancer Ther. 2021 Jun;21(6):657-671.
[4] Marret G, et al. Expert Opin Biol Ther. 2019 Sep;19(9):871-877.
[5] McLachlan J, et al. Clin Oncol (R Coll Radiol). 2017 Mar;29(3):153-160.
[6] Chung, H. C. et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 37, 1470–1478 (2019).
[7] Tewari, K. S. et al. Survival with cemiplimab in recurrent cervical cancer. N. Engl. J. Med. 386, 544–555 (2022).
[8] Naumann, R. W. et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkMate 358 trial. J. Clin.Oncol. 37, 2825–2834 (2019).
[9] Vergote I, et al. NEJM. 2024 Jul; 391(1):44-55. ADC, antibody-drug conjugate; CC, cervical cancer; DAR, drug-antibody ratio; mPFS, median progression-free survival; ORR, objective response rate; PD-L1, programmed cell death-ligand 1; TME, tumour microenvironment; Top1, topoisomerase 1.
关于 复宏汉霖
复宏汉霖 (2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已在全球获批上市10款产品,4个上市申请分别获中国药监局和欧盟EMA受理。自2010年成立以来, 复宏汉霖 已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,公司商业化生产基地已相继获得中国、欧盟和美国GMP认证。
复宏汉霖 前瞻性布局了一个多元化、高质量的产品管线,涵盖约50个分子,并全面推进基于自有抗PD-1单抗H药汉斯状?的肿瘤免疫联合疗法。截至目前,公司已获批上市产品包括全球首个获批一线治疗小细胞肺癌的抗PD-1单抗汉斯状?(斯鲁利单抗,欧洲商品名:Hetronifly?)、自主研发的中美欧三地获批单抗生物类似药汉曲优?(曲妥珠单抗,美国商品名:HERCESSI?,欧洲商品名:Zercepac?)、国内首个生物类似药汉利康?(利妥昔单抗)、地舒单抗生物类似药Bildyos?和Bilprevda?,以及帕妥珠单抗POHERDY?。公司亦同步就19个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
Henlius'' PD-L1 ADC HLX43 Cervical Cancer Data Debuts as Proffered Paper at 2025 ESMO Asia
The encouraging efficacy observed across multiple solid tumors corroborates the broad-spectrum anti-tumor potential of HLX43
Phase 2 clinical data of HLX43 in advanced cervical cancer presented in Proffered Paper Session at the 2025 ESMO Asia
Encouraging preliminary efficacy was observed, with an ORR of 41.4% and DCR of 82.8% in the overall population, rising to 70.0% and 100% in the 3 mg/kg group, alongside a manageable safety profile
Shanghai, China, December 5, 2025—Shanghai Henlius Biotech, Inc.(2696.HK) today announced that the results from a phase 2 proof of concept (POC) study of Henlius’ PD-L1 antibody-drug conjugate (ADC) HLX43 in recurrent/metastatic cervical cancer were first presented in Proffered Paper Session at the 2025 ESMO Asia Congress. The results demonstrated encouraging antitumor activity, providing further evidence of its broad-spectrum antitumor activity, previously observed in non-small cell lung cancer and thymic carcinoma.
Promising CC Data Add to Growing Evidence for Broad-Spectrum Potential
Cervical cancer (CC) and other gynaecological cancer pose a serious threat to women''s health [1-2], particularly with poor prognosis in advanced-stage patients. Approximately 30% of advanced CC patients experience disease recurrence after first-line combination therapy [3], with a 5-year survival rate of about 17% [4]. The objective response rate (ORR) of second-line chemotherapy, including docetaxel, is only around 13.2% [5]. Although PD-1 inhibitors and the tissue factor (TF)-targeting antibody-drug conjugate (ADC) tisotumab vedotin have been approved, their efficacy in recurrent/metastatic CC after prior treatment remains limited, with ORR typically below 18% [6-9]. There is an urgent need for more effective therapeutic options with greater clinical benefits.
This open-label, randomised, multicentre phase 2 study was led by Academician Jinming Yu from the Shandong Cancer Hospital. Patients with histologically confirmed recurrent/metastatic CC who had previously failed, intolerant to, or contraindicated for standard first-line therapy were randomised 1:1:1 to receive HLX43 at 2 mg/kg, 2.5 mg/kg, or 3 mg/kg every 3 weeks. The primary endpoints were investigator-assessed objective response rate (ORR) and progression-free survival per RECIST v1.1. Secondary endpoints included other efficacy measures, safety, pharmacokinetics, immunogenicity, and biomarker explorations.
By the data cutoff date of September 1, 2025, 30 patients were enrolled and randomized to receive HLX43 at 2 mg/kg (n=10), 2.5 mg/kg (n=10) and 3 mg/kg (n=10). Over 80% of the patients had a PD-L1 combined positive score ≥ 1. The median line of prior antitumor therapy was 2.0 (range, 1–4). All patients received platinum-based chemotherapy, 60% received targeted therapy, approximately 50% received immunotherapy, and the median follow-up time was 3.5 months.
Among the 29 response evaluable patients, investigator-assessed ORR was 41.4% and disease control rate (DCR) was 82.8%. ORR, and DCR for the 3 mg/kg dose group was 70.0%, and 100%, respectively. The median Progression-Free Survival (PFS) has not yet been reached.
Subgroup analysis demonstrated that HLX43 exhibited promising preliminary efficacy in PD-L1 positivive(CPS≥1) advanced cervical cancer patients, with an ORR of 45.8% and a DCR of 87.5%, respectively. Notably, a signal of efficacy was also observed in PD-L1 negative (CPS <1) patients, with one patient in the 2 mg/kg dose group achieving partial response (PR). However, as the sample size of this study was limited and the population predominantly consisted of PD-L1 positive patients (>80%), these findings are preliminary. Further investigation in larger, randomized controlled trials is required to adequately characterize the efficacy of HLX43 in the PD-L1 negative subgroup.
In terms of safety, grade ≥3 treatment-related adverse events were reported in 18 (60.0%) patients, most commonly neutrophil count decreased (30.0%), anemia(30.0%) and lymphocyte count decreased (23.3%). Dose reductions due to TRAEs were reported in 6 patients (20.0%), with no TRAE-related treatment discontinuations and TRAE-related death. Immune-related adverse events were observed in 7 (23.3%) patients, primarily including endocrine disorder and rash. No grade 3 or higher immune-related adverse events (irAEs) were reported. This finding is supportive of an immunomodulatory mechanism of action for HLX43 and indicates a manageable and favorable immune-related safety profile.
HLX43, particularly at 3 mg/kg, exhibited promising efficacy and manageable safety in patients with previously treated recurrent/metastatic CC. Further investigation of HLX43 is warranted.
Compelling Efficacy in Lung Cancer Lays Foundation for Blockbuster Profile
HLX43 is a potential best-in-class as well best-in-disease broad-spectrum anti-tumor ADC candidate targeting PD-L1, which exhibits dual mechanisms integrating immune checkpoint blockade and payload-mediated cytotoxicity. Preclinical data has shown that, HLX43 has good anti-tumor effects and a favorable tolerability profile in NSCLC, cervical cancer (CC), esophageal squamous cell carcinoma (ESCC), and other tumor types that were PD-1/L1 mAb-resistant. The results from the phase 1 clinical trial of HLX43 were released at the 2025 ASCO Annual Meeting and 2025 WCLC, demonstrating manageable safety profile and encouraging efficacy in various solid tumors especially in patients with NSCLC, including squamous and non-squamous NSCLC patients (sqNSCLC and nsqNSCLC), patients with or without EGFR mutation, patients with or without brain metastasis, and PD-L1 positive or negative patients.
The company is currently accelerating the clinical development of HLX43. To date, over 500 patients enrolled globally for HLX43, including more than 270 patients with NSCLC. With its efficacy in later-line NSCLC being validated, Henlius plans to initiate additional Phase 3 clinical studies for HLX43 in lung cancer. These trials will encompass a second-line, head-to-head study comparing HLX43 against docetaxel, as well as explorations in first-line treatment and neoadjuvant therapy regimens. Meanwhile, as the first PD-L1 ADC developed for thymic carcinoma globally, HLX43 is advancing its international multi-center clinical trials concurrently in China, the U.S., Australia, etc. In October 2025, based on the compelling preliminary efficacy data from later-line settings in thymic carcinoma (TC), the U.S. FDA granted Orphan Drug Designation (ODD) to HLX43 for the treatment of Thymic epithelial tumors (TETs), highlighting the drug''s potential to address the significant unmet need for ADC therapies in this disease.
In addition to NSCLC and TC, Henlius is actively exploring HLX43''s therapeutic potential in various solid tumors. The company has initiated about 10 clinical studies for HLX43, covering CC, ESCC, head and neck squamous cell carcinoma (HNSCC), nasopharyngeal cancer (NPC), colorectal cancer (CRC), gastric or gastroesophageal junction (G/GEJ) adenocarcinoma, pancreatic ductal adenocarcinoma (PDAC) and hepatocellular carcinoma (HCC). Proof-of-concept (PoC) results in ESCC, NPC and G/GEJ are also expected to be released on the upcoming international academic conferences including the ASCO Gastrointestinal (GI) Cancers Symposium, ASCO and ESMO. Building on its intrinsic immuno-oncology (IO) activity, Henlius is exploring combination therapies, such as with the in-house anti-EGFR antibody HLX07, to maximize HLX43''s clinical value.
Guided by its commitment to address unmet medical needs, Henlius will continue to advance its core pipeline including HLX43 to deepen the differentiated therapeutic potential of our products, accelerate the delivery of greater clinical value, and ultimately deliver more breakthrough treatment options to patients worldwide.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases and ophthalmic diseases. To date, 10 products have been approved for marketing across multiple countries and regions, and 4 marketing applications have been accepted for review in China and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centre and Shanghai-based commercial manufacturing facilities certificated by China, the EU and U.S. GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering about 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as the backbone. To date, the company''s launched products include HANSIZHUANG (serplulimab, trade name: Hetronifly? in Europe), the world’s first anti-PD-1 mAb for the first-line treatment of SCLC, HANQUYOU (trastuzumab, trade name: HERCESSI? in the U.S., Zercepac? in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANLIKANG (rituximab), the first China-developed biosimilar, denosumab Bildyos? and Bilprevda?, and pertuzumab Poherdy?. What’s more, Henlius has conducted over 30 clinical studies for 19 products, expanding its presence in major markets as well as emerging markets.
To learn more about Henlius, visit https://www.henlius.com/en/index.html and connect with us on LinkedIn at https://www.linkedin.com/company/henlius/.